
Shares of Ipca Laboratories Ltd. gained over five percent on Thursday after the company announced that its Piparia manufacturing facility in Silvassa got a Voluntary Action Indicated (VAI) status from the United States Food and Drug Administration (USFDA).
The company said that the US drug regulator inspected Piparia formulations manufacturing facility from April 18 to April 26, which resulted in 3 observations issued with a Form 483 by USFDA.
Now, the pharma company has received a communication from USFDA that they have determined the inspection classification of this manufacturing facility as" VAI", IPCA Laboratories said in a filing to the stock exchanges.
Based on the inspection, the Piparia facility is considered to be in a minimally acceptable state of compliance with current good manufacturing practice (CGMP).
As per the USFDA website, the VAI inspection classification indicates that, although investigators found and documented objectionable conditions during the inspection, the drug regulator will not take or recommend regulatory or enforcement action because the objectionable conditions do not meet the threshold for action at this time.
Earlier, Ipca Laboratories' Pithampur unit in Madhya Pradesh was inspected by the USFDA from June 15 to June 23, after which the US federal agency issued eight observations to the firm.
The USFDA observed that the pharma company’s plant failed to review discrepancies in batch distribution and did not establish time limits for completion of the production phase to ensure quality.
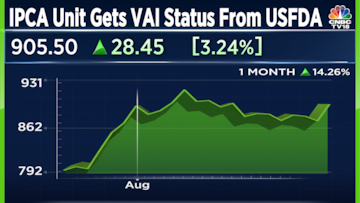
Shares of IPCA Labs are trading 3.2 percent higher at Rs 905.25. The stock has gained 8.2 percent this year so far, underperforming the Nifty Pharma index.



