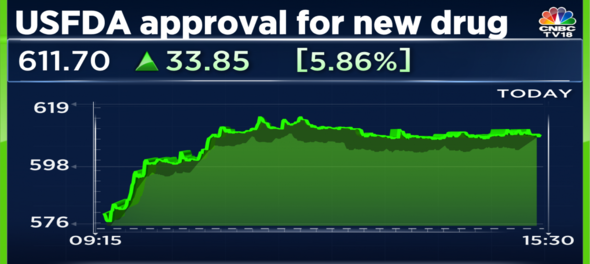
Shares of Alembic Pharmaceuticals gained over 5.5 percent on Wednesday after the drug maker received final approval from the United States Food and Drug Administration (USFDA) for Mesalamine extended-release capsules UPS, 0.375g.
Mesalamine extended-release capsules are used for the treatment of ulcerative colitis and to prevent the remission of the disease in adults.
According to IQVIA data for the 12-month period ending June 2022, Mesalamine extended-release capsules USP, 0.375 g, have an estimated market size of US$ 133 million.
Alembic Pharma has a cumulative total of 174 ANDA approvals, including 150 final authorisations, from USFDA. Alembic Pharmaceuticals is a vertically integrated research and development pharmaceutical company, which manufactures and markets generic pharmaceutical products globally.
Alembic Pharmaceuticals, on November 1, also received another USFDA nod for its first injectable product from its General Sterile Facility - Glycopyrrolate Injection USP. The injection is used during surgeries and in the treatment of peptic ulcers.
Shares of Alembic Pharmaceuticals ended at Rs 610.50, 5.65 percent higher on Wednesday.
The company will declare its September quarter results on November 11.
Also Read: Alembic Pharmaceuticals declines as US regulator issues observations to Panelav's facility
(Edited by : Rukmani Krishna)
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!


Rapido offers free rides to voters to polling stations on May 13 in Hyderabad, 3 other cities
May 6, 2024 5:49 PM
Lok Sabha elections 2024: Seats to date, all you need to know about third phase of voting
May 6, 2024 4:49 PM
Concerns on low voter turnout a "myth"; absolute number of voters correct way to analyse: Report
May 6, 2024 2:57 PM
Haryana Lok Sabha elections 2024: A look at JJP candidates
May 6, 2024 2:26 PM

