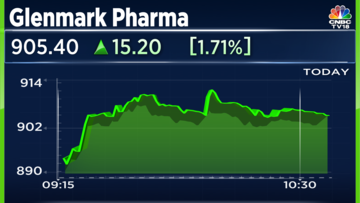
Glenmark Pharmaceuticals Limited shares gained more than 2% in morning trade on Thursday, January 25, after the company announced that its subsidiary has signed a licence agreement to market cancer drugs in India and other regions.
In a stock exchange filing, Glenmark Pharma said its subsidiary Glenmark Specialty SA signed a licence agreement with Jiangsu Alphamab Biopharmaceuticals and 3D Medicines for KN035 Envafolimab, which is used to treat cancer patients, in India and other geographies. Apart from India, the other geographies include Asia Pacific, Middle East, Africa, Russia, CIS and Latin America, the company said.
Under the agreement terms, Glenmark Specialty gains exclusive rights to develop, register and commercialise Envafolimab for oncology in the specified territory.
Jiangsu Alphamab will be responsible for manufacturing KN035 within and outside the territory.
Further, Jiangsu Alphamab, as the exclusive supplier, will receive a low double-digit million USD amount pre-launch, along with triple-digit million USD milestone payments linked to sales performance throughout the agreement. Additionally, a royalty fee, ranging from single to double digits, will be paid based on net sales.
Envafolimab addresses a prevalent genetic signature, deficient MisMatch repair, found in about 16% of cancer patients across 13 tumor types.
"This is an important milestone for Glenmark, as through this transformational deal, we gain access to the first recombinant humanised single domain antibody against PD-L1 in a Sub-Q formulation for a wide territory globally. We are excited at the opportunity to take this innovative immuno-oncology product to cancer patients across the emerging markets,” Glenn Saldanha, Chairman and Managing Director, Glenmark Pharmaceuticals, said.
Envafolimab received approval in China from the National Medical Products Administration (NMPA) in November 2021. It's the first global subcutaneous injection PD-L1 inhibitor to treat adults with previously treated microsatellite instability-high or deficient MisMatch repair advanced solid tumors. The product has benefited over 30,000 patients in China.
In the United States, Tracon Pharma is developing Envafolimab.
Shares of Glenmark Pharmaceuticals were trading 1.83% higher at ₹906.45 apiece at 10.40am on Thursday, January 25.
Also Read: Bajaj Auto Q3 Results: The bull vs bear case
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!



