
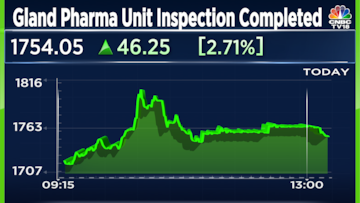
Gland Pharma shares surged up to 6.5% on Friday after the company announced that the United States Food and Drug Administration (USFDA) completed the inspection of its Pashamylaram facility in Hyderabad.
Gland Pharma has received the Establishment Inspection Report (EIR) from the US drug regulator indicating the closure of the inspection, the company stated in an exchange filing.
The USFDA conducted a pre-market inspection of the Pashamylaram facility from August 23 to 26, 2023. The inspection was conducted to assess the facility's compliance with the USFDA's Quality System/Current Good Manufacturing Practice (CGMP) regulations for medical devices.
The Pashamylaram facility, commissioned in July 2012, started manufacturing products for the US market in September 2016.
Gland Pharma shares surged 6.37% to hit a high of ₹1817.15 on BSE following the announcement. Trading volume surged by more than fourfold on the bourse.
Gland Pharma is one of the largest injectable-focused companies, with a global footprint across 60 countries, including the US, Europe, and Canada.
It operates primarily under a business-to-business (B2B) model. It has a wide range of injectables, including vials, ampoules, pre-filled syringes, lyophilized vials, dry powders, infusions, oncology, and ophthalmic solutions.
The company reported a 19.56% year-on-year (YoY) fall in consolidated net profit to ₹194 crore for the July-September quarter of FY 2023-24 compared to ₹241.2 crore a year ago. Its revenue came in at ₹1,373.4 crore in Q2 of FY24, up 31.52% over last year's ₹1,044.4 crore.
Last week, the company announced it had received a tentative approval from the USFDA for Angiotensin II Injection, 2.5 mg/mL Single Dose Vial. The injection is used to treat low blood pressure in adults.
Gland Pharma shares were trading 2.82% higher at ₹1,756.40 apiece on BSE at 11.41am.
(Edited by : Shloka Badkar)
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!


Prajwal Revanna's father in custody for alleged kidnapping and sexual abuse
May 4, 2024 7:53 PM
Delhi, Indore, Surat and Banswara — why these are the most challenging domains for Congress internally
May 4, 2024 1:53 PM
Congress nominee from Puri Lok Sabha seat withdraws, citing no funds from party
May 4, 2024 12:00 PM
Lok Sabha Polls '24 | Rahul Gandhi in Rae Bareli, why not Amethi
May 4, 2024 9:43 AM

