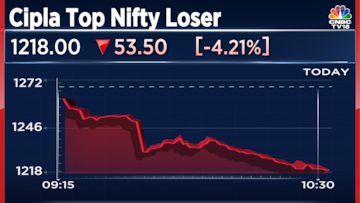
Shares of Cipla Ltd., the Mumbai-based drugmaker are the top losers on the Nifty 50 index on Thursday after the United States Food & Drug Administration (USFDA) made public the warning letter it issued to the company's Pithampur unit on November 20.
CNBC-TV18 has managed to access the warning letter issued which includes the data integrity issues highlighted by the regulator, with product complaints and microbial contamination.
Additionally, the letter mentions similar and repeat observations at the Goa site. The Goa unit is already under a warning letter from the USFDA.
The USFDA has asked Cipla to seek help of third-party consultants to address these issues.
Cipla's Pithampur facility was inspected by the USFDA between Februrary 6 - February 17, 2023, post which it had issued a form 483 with eight observations. The said plant manufacturers respiratory and oral products for the company.
Going by the US drug regulator’s classification, Official Action Indicated (OAI) generally means objectionable conditions were found and regulatory or administrative actions by USFDA are indicated during inspections.
This also means that new approvals from the site will be put on hold for now. The delay in Cipla's inhaler gAdvair is already factored into the consensus estimates and the stock price.
The street was anticipating the warning letter after the form 483 issuance.
Along with gAdvair, the launch of gAbraxane also gets pushed to financial year 2025.
Vohra is optimistic on Advair generic. He says while the opportunity has diluted from a time based value it has not changed from a market perspective and he doesnt expect more players coming into the Advair generic market.
Cipla's shares are now trading 4% lower at 1,220. The stock is still up 14% so far in 2023.
In an interview with CNBC-TV18,
Nimish Mehta, Founder Partner of Research Delta Advisors, delved into a detailed discussion about Cipla.
(With Inputs From Ekta Batra.)
(Edited by : Hormaz Fatakia)
First Published: Nov 23, 2023 10:31 AM IST