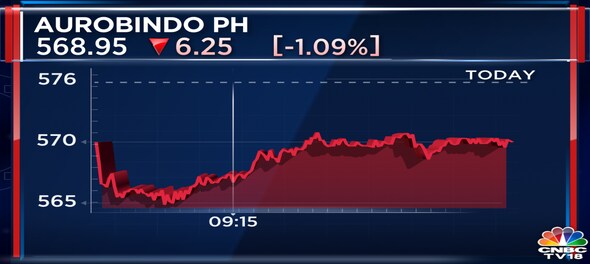
Shares of Aurobindo Pharma fell as much as 2 percent on Wednesday after the United States Food and Drug Administration (USFDA) issued three observations to the company’s unit in Andhra Pradesh.
At 9:20 am, shares of the company were trading 1.3 percent lower at Rs 567.55 on the BSE.
Also Read:
The USFDA inspected the company's Unit XI, an Active Pharmaceutical Ingredient non-antibiotic manufacturing facility at Pydibhimavaram in Andhra Pradesh's Srikakulam district, from July 25 to August 2, 2022.
“We have been issued a 'Form 483' with three observations, and none of these observations is related to data integrity,” Aurobindo Pharma said in an exchange filing.
This comes after the unit was classified as official action indicated on May 17, 2019, and was subsequently given a warning letter dated June 20, 2019.
Official Action Indicated (OAI) means objectionable conditions were found, and regulatory and administrative sanctions by the USFDA are indicated.
The company had responded to the warning letter and carried out the committed corrections, it had said in the filing.
“We will respond to the USFDA within the stipulated timeline and work closely with the regulators to address the observations at the earliest,” the company added.
The pharma company had earlier been served with a warning letter by the capital markets regulator, Securities Exchange Board of India (SEBI), regarding insufficient disclosures for another unit. The letter observed that the company had disclosed very limited and restricted information and did not consider observations of the USFDA as serious.
Aurobindo Pharma is India's fifth largest drug maker by sales, after Sun Pharma, Dr Reddy's Laboratories, Cipla and Lupin.
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!


Lok Sabha Election Phase 2: Experts decode the key trends and issues in key battleground states
Apr 26, 2024 11:53 PM
2024 Lok Sabha Election | Which way the wind blows in the second phase
Apr 26, 2024 6:09 PM

