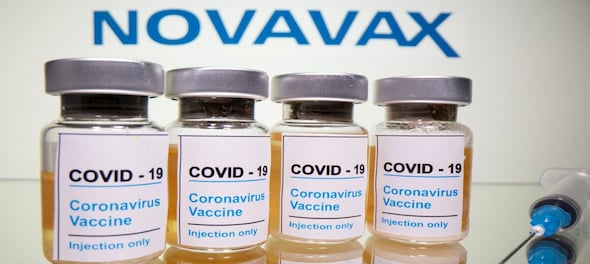
Novavax's updated vaccine has been granted emergency-use authorization by the World Health Organization (WHO) for active immunization to prevent COVID-19 in individuals aged 12 and older, the company said on Tuesday.
The single-target vaccine is aimed at the XBB.1.5 Omicron subvariant of the coronavirus, which was dominant in the U.S. for much of this year but has since been overtaken by other variants as the virus evolves.
Novavax missed out on the COVID-19 vaccine windfall, which benefited rivals, due to manufacturing issues that delayed its filing for approval during the peak of the pandemic.
The Maryland-based biotech's updated COVID shot, which was authorized in the U.S. in October, uses a more traditional protein-based technology than the mRNA-based vaccines by rivals Pfizer and Moderna.
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!


Delhi, Indore, Surat and Banswara — why these are the most challenging domains for Congress internally
May 4, 2024 1:53 PM
Congress nominee from Puri Lok Sabha seat withdraws, citing no funds from party
May 4, 2024 12:00 PM
Lok Sabha Polls '24 | Rahul Gandhi in Rae Bareli, why not Amethi
May 4, 2024 9:43 AM

