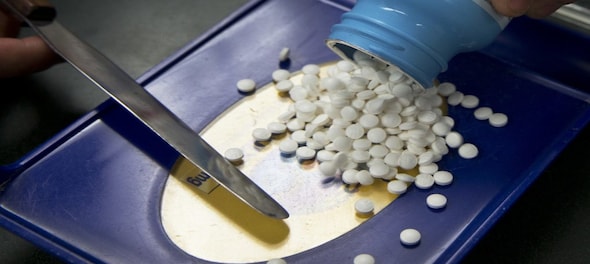
In a major relief to pharmaceutical companies, the government has decided to do away with a clause in the draft clinical trial rules that binds the entity initiating trial to pay 60 percent of the compensation upfront in case of death or permanent physical disability of the patient, Mint reported.
However, the companies must adhere to a timeline and cannot take forever to pay the compensation, the report said citing a person privy to the discussions.
The earlier proposal made by the Union health ministry included a mandate to pay 60 percent of the compensation within 15 days if a person dies or suffers a permanent disability, said the report.
According to India’s drugs regulatory authority Central Drugs Standard Control Organisation (CDSCO), major pharmaceutical companies conducting medical trials in India include global giants such as Novartis, Pfizer, Merck KGaA, and AstraZeneca, as well as Indian drug firms such as Torrent Pharmaceuticals Ltd, Sun Pharmaceuticals Industries Ltd, and Biocon Ltd, said the report.
Have you signed up for Primo, our daily newsletter? It has all the stories and data on the market, business, economy and tech that you need to know.
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!


PM Modi, Rahul Gandhi election rallies in Delhi today: Here are the routes to avoid
May 18, 2024 11:28 AM
Celebrity Kangana vs 'royal' Vikramaditya on Himachal's Mandi seat: Clash of richest titans
May 18, 2024 11:11 AM
2024 Lok Sabha Elections | Will Amethi and Rae Bareli see the rise of Priyanka Gandhi as a dominant political figure
May 18, 2024 8:59 AM
Lok Sabha Election 2024: I.N.D.I.A. bloc to hold rally at Mumbai's BKC today
May 17, 2024 5:18 PM

