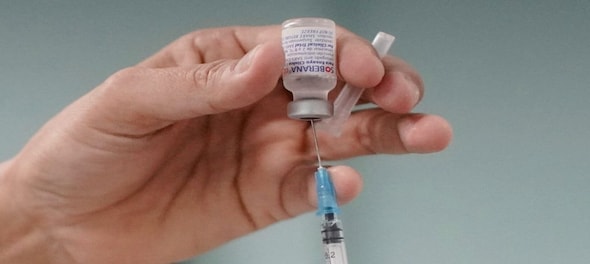
India's Drug Controller General of India (DCGI) has granted approval to Gennova Biopharmaceuticals for its mRNA-based Omicron-specific booster vaccine.
The vaccine, named GEMCOVAC®-OM, is intended for individuals aged 18 years and older as a booster dose. It can be safely administered to individuals who have already received two doses of either Covaxin or Covishield.
The vaccine is administered intradermally using a needle-free device, and it remains stable at temperatures between 2-8 °C, due to which it does not require ultra-cold chain infrastructure used for other approved mRNA-based vaccines, making it easy for deployment pan India.
The Department of Biotechnology (DBT) has facilitated establishing the vaccine manufacturing for developingthe platform technology from proof of concept till Phase I clinical trial of the prototype mRNA-based vaccine developed against the Wuhan strain.
Union Minister Jitendra Singh commended the efforts and said, "I take great pride in DBT fulfilling its mission yet again – enabling technology-driven entrepreneurship through creating this indigenous mRNA-platform technology. We have always supported technology-driven innovation towards the creation of a 'future-ready' technology platform in line with the Prime Minister’s vision of Aatmanirbharta."
He also said, "Infrastructure to deploy vaccine in India, including LMICs, at 2‑8°C exist today & this innovation is tailored for the existing established supply-chain Infrastructure. The vaccine does not need ultra-low temperature conditions for transport and storage."
Gennova Biopharmaceuticals CEO Dr. Sanjay Singh, said India has now developed two mRNA vaccines against COVID-19.
"I am proud that my team has worked tirelessly over the last two years to develop the nation's first mRNA vaccine. This is a team effort & without the guidance of the Subject Expert Committee of CDSCO and the Vaccine Expert Committee of BIRAC monitoring the project, it would not have been possible."
(Edited by : Pradeep John)
First Published: Jun 20, 2023 5:28 PM IST
Check out our in-depth Market Coverage, Business News & get real-time Stock Market Updates on CNBC-TV18. Also, Watch our channels CNBC-TV18, CNBC Awaaz and CNBC Bajar Live on-the-go!


Rapido offers free rides to voters to polling stations on May 13 in Hyderabad, 3 other cities
May 6, 2024 5:49 PM
Lok Sabha elections 2024: Seats to date, all you need to know about third phase of voting
May 6, 2024 4:49 PM
Concerns on low voter turnout a "myth"; absolute number of voters correct way to analyse: Report
May 6, 2024 2:57 PM
Haryana Lok Sabha elections 2024: A look at JJP candidates
May 6, 2024 2:26 PM

