Drug firm Zydus Lifesciences Ltd on Monday (June 19) said the company has received final approval from the United States Food and Drug Administration (USFDA) for minocycline hydrochloride extended-release tablets USP, 55 mg, 65 mg, and 115 mg.
Minocycline hydrochloride extended-release tablets are used to treat moderate to severe acne in people 12 years and older. Minocycline belongs to a class of drugs known as tetracycline antibiotics, it said in an exchange filing.
The product will be manufactured at the group’s formulation manufacturing facility in Moraiya, Ahmedabad.
Minocycline hydrochloride extended-release tablets USP, 55 mg, 65 mg, and 115 mg had annual sales of $0.7 million in the United States according to IQVIA MAT April 2023.
The group now has 372 approvals and has so far filed over 442 (as of March 31, 2023) abbreviated new drug applications (ANDAs) since the commencement of the filing process in FY 2003-04.
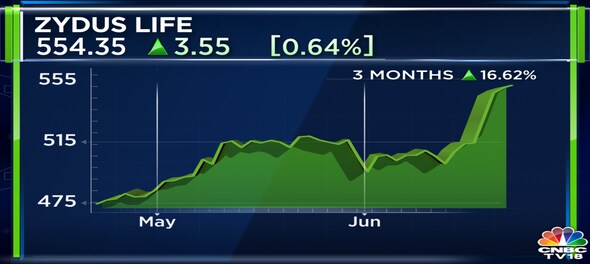
Shares of Zydus Lifesciences Ltd ended at Rs 554.35, up by Rs 3.55, or 0.64 percent on the BSE.



